1.Map of Coronavirus-East Coast Highest Concentration.
NY Times. https://www.nytimes.com/section/todayspaper?redirect_uri=https%3A%2F%2Fwww.nytimes.com%2F
Washington Post-Children and Corona
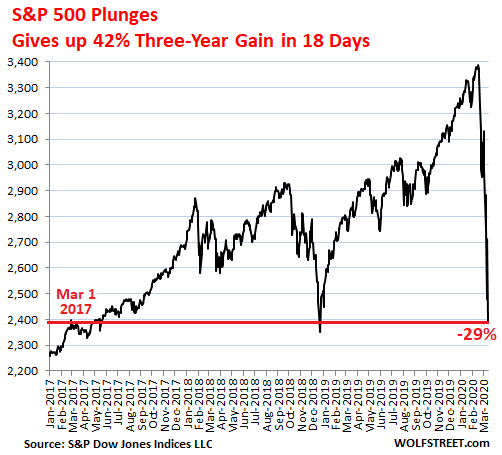
2.S&P Plunged 29% in 18 Trading Days.
The S&P 500 index has now plunged 29% in the 18 trading days since the peak in February 19 and is below where it had first been on March 1, 2017 – which was over three years ago. In other words, the S&P 500 unwound three years’ worth of gains in 18 trading days.
S&P 500 Plunged Most Since 1987, Gave Up in 18 Days the 42% Gains of Past 3 Years. Boeing Shares Collapsed-Wolf Richter
20% Correction was 22 Days Vs. Historical Average 296 Days
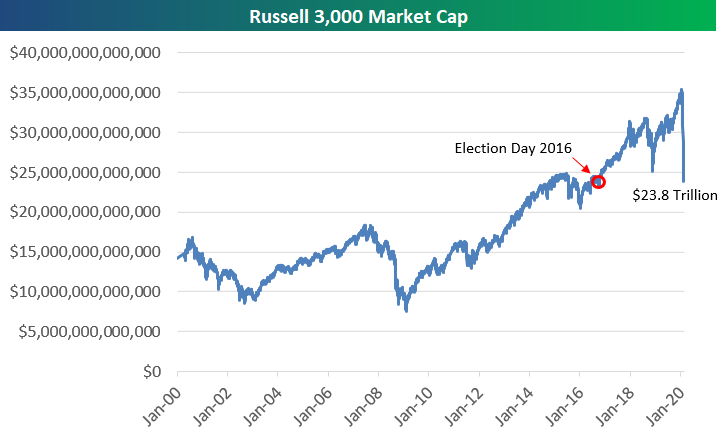
3.Selloff Erases All of US Market Cap Gains Since Election Day 2016
Bespoke
With the US stock market down nearly 7% yet again today, the total market cap of US companies as measured by the Russell 3,000 has fallen $11.5 trillion in less than a month. On February 19th, total US market cap was just over $35 trillion. It’s at $23.8 trillion as of this morning.
What makes this drop even more noteworthy is that $23.8 trillion was the market cap of US companies on Election Day 2016. At this point in time, all of the market cap gains seen since President Trump’s election victory have been wiped out. Visit our membership options page to learn more about Bespoke’s research.
4.International Markets Have Worst Day Ever Including 2008 Crisis Before Today
MSCI World Index Record Down Day
https://blogs.wsj.com/dailyshot/
5.S&P 500 Performance Around U.S. Recessions…
The U.S. markets are already at average decline for a recession
And the S&P 500 performance around US recessions
From Dave Lutz at Jones Trading
6.Pullbacks During 11 Year Bull….Corona Virus Hits Bear Level.
S&P 500 Snapshot: Officially a Bear Market–by Jill Mislinski, 3/12/20
7..U.S. Only Has 950,000 Hospital Beds.
https://www.statista.com/statistics/185860/number-of-all-hospital-beds-in-the-us-since-2001/
8.Torsten Slok on Doctors and Nurses.
The capacity of the healthcare system in different countries is becoming very important, see charts below and also the piece here from my colleagues in healthcare research: Cross Healthcare Research – DB’s Initial Covid-19 Projections
———————————————–
Let us know if you would like to add a colleague to this distribution list.
Torsten Sløk, Ph.D.
Chief Economist
S. Korea Testing 15,000 Citizens Per Day CORONAVIRUS Testing 1…2…3…
Francis Scialabba
| One of the major criticisms of the U.S. government’s response to the coronavirus pandemic has been the woeful lack of testing capacity for the disease. Almost two months after the first U.S. case was announced, the NIH’s top infectious disease expert said, “The system is not really geared to what we need right now…it is a failing, let’s admit it.” That’s slowly being remedied. Yesterday, the FDA gave emergency clearance to a coronavirus test developed by the Swiss diagnostics giant Roche. This thing is Speedy Gonzales:It can test patients 10x faster than existing tests.Using Roche’s automated machines, it can produce results in 3.5 hours.The most powerful system can test up to 4,128 patients a day.Roche Diagnostics CEO Thomas Schinecker said getting a new test developed and approved typically takes years. An “emergency team” started working on this one in January. Other signs of lifeYesterday, the Trump administration made a few other moves to ramp up testing. First, someone is now finally in charge of the government’s testing effort—the assistant secretary of health, Brett Giroir. Next, the Department of Health and Human Services said it was funding two companies, DiaSorin Molecular and Qiagen, to push them along in developing diagnostic tests.The agency said these tests may be able to detect coronavirus in “approximately one hour.” Looking aheadDr. David Kessler, a former FDA commissioner, explained in the NYT yesterday that there are a few ways the private sector can help expand testing capacity: Delivery companies like FedEx, UPS, and Amazon can transfer specimens from mobile collection sites to labs.Then, bring in the big guns: Apple, Microsoft, Google, and Salesforce can supply the data management systems to take those specimens and turn them into useful information. Bottom line: “There’s no reason the testing infrastructure can’t be up and running in seven days so that every person in America who needs a test can be tested,” Kessler wrote. |
Gates-funded program will soon offer home-testing kits for new coronavirus —By Sandi Doughton
Seattle Times staff reporter
Testing for the novel coronavirus in the Seattle area will get a huge boost in the coming weeks as a project funded by Bill Gates and his foundation begins offering home-testing kits that will allow people who fear they may be infected to swab their noses and send the samples back for analysis.
Results, which should be available in one to two days, will be shared with local health officials who will notify those who test positive. Via online forms, infected people can answer questions about their movements and contacts, making it easier for health officials to locate others who may need to be tested or quarantined, as well as to track the virus’ spread and identify possible hot spots.
The goal is to eventually be able to process thousands of tests a day, said Scott Dowell, leader of coronavirus response at the Bill & Melinda Gates Foundation. The project is ramping up as quickly as possible, but it’s not clear exactly when it will launch, he added. Among other things, software needs to be upgraded to handle the expected crush of requests, and a detailed questionnaire finalized for people who request tests.
“Although there’s a lot to be worked out, this has enormous potential to turn the tide of the epidemic,” Dowell said.
10..How does the coronavirus test work? 5 questions answered
Associate Professor of Biology, Rochester Institute of Technology
The U.S. government is fighting to contain and slow down the spread of the coronavirus. Testing is central to these efforts. Molecular biologist and viral researcher Maureen Ferran answers some basic questions about how these diagnostic tests work – and if there are enough to go around.
Who gets tested for the virus?
Currently there are two main reasons someone would be tested for the coronavirus: having symptoms or exposure to an infected person.
The main symptoms of COVID-19, the disease caused by the coronavirus SARS-CoV-2, are fever, dry cough and shortness of breath. These look a lot like the flu and the common cold, so it takes a physician to determine if testing for the virus is necessary.
Initially, the Centers for Disease Control and Prevention recommended testing only people with symptoms and who had potentially been exposed to the virus. But to the surprise of public health officials, several of the first people in the U.S. who tested positive for the virus had no obvious exposure. This development suggested that the virus was being transmitted locally, meaning it was spreading from person to person easily and/or that people may have been transmitting the virus without experiencing serious symptoms.
In response, on March 4 the CDC changed its recommendations to allow anyone with COVID-19-like symptoms to be tested as long as a doctor approved the request. Since the number of available tests is limited, the CDC is encouraging physicians to minimize unnecessary testing and consider a patient’s exposure risks before ordering tests.
As of writing this, there are no specific treatments available for COVID-19, but that does not mean testing is pointless. Perhaps most importantly, testing is done so that infected patients can be quarantined and the spread of the virus slowed. Another benefit of testing is that it lets public health workers build a more accurate picture of the number of cases and how the virus is spreading in the population.
What it is like to get tested?
For a patient, the process of being tested for the virus is easy and can potentially be done almost anywhere. It typically involves taking a swab from deep in a patient’s nasal cavity to collect cells from the back of the nose. The sample is then sent to a lab, where it will be tested to determine if the patient’s cells are infected with the virus. The same process is used to collect a sample from a patient who is tested for flu.
How does the test work?
While collecting a sample is easy, actually determining whether a person is infected with the coronavirus is much more complicated. The current method looks for the virus’s genetic material (RNA) in a patient’s cells.
In order to detect the presence of RNA in the patient’s sample, labs perform a test called reverse-transcription polymerase chain reaction. This method first converts any viral RNA to DNA. Then the DNA is replicated millions of times until there are enough copies to detect using a specialized piece of equipment called a quantitative PCR instrument.
If genetic material from the virus is found in the sample, then the patient is infected with the virus.
It takes 24-72 hours to get the results of a test. During the early ramp-up of testing, there were some concerns about the test’s accuracy after one study found 3% of tests in China came back negative when the samples were actually positive. But this type of genetic test is generally very accurate – more so even than rapid flu tests – and the benefits of testing outweigh the risk of an error.
As the virus spread, the testing capacity needed to grow, and fast. AP Photo/Andrea Casalis
Does the US have enough tests?
The availability of tests has been a big issue. Prior to Feb. 29, the CDC was the only place approved by the FDA to develop, produce and process tests. However, as the number of suspected cases climbed and doctors approved more people for testing, demand to be tested soared.
The test for the coronavirus requires a kit, specialized equipment and specially trained personnel. Faulty and slow development of test kits and the initial requirement that all tests be processed at the CDC contributed to the slow rollout across the U.S.
As pressure on the federal government to make tests available increased, the FDA announced a new policy on Feb. 29 that made it easier for commercial and academic laboratories to develop their own tests and allowed other certified labs to test patient samples.
Integrated DNA Technologies, a CDC contractor, shipped 700,000 tests to commercial, academic and health care laboratories on March 6. Quest Diagnostics and LabCorp, two large commercial test manufacturers, started making their own test kits, which became available on March 9. Many companies, hospitals and other institutions are now racing to develop more tests to diagnose COVID-19.
On March 10, Alex Azar, secretary of Health and Human Services, announced that 2.1 million testing kits are now available and more than 1 million have shipped to certified labs for testing. Millions more are expected to ship out this week.
Does everyone really need to be tested?
Realistically, it isn’t feasible to test everyone who is sick in the U.S. Therefore, most health officials believe it is important to prioritize the testing of people who need it the most: those at high risk such as health care workers who have been in contact with COVID-19 patients; symptomatic people in areas with high infection rates; and people 65 years of age and older with chronic health issues, such as heart disease, lung disease or diabetes. As more tests become available, it will be possible to test more people.
There’s also a need to develop faster tests that do not require special equipment and personnel. Testing allows experts to better understand how the outbreak is progressing and try to predict the impact the virus will have on society.
As with all outbreaks, this pandemic will end. In the meantime, however, people need to wash their hands and try to minimize their risk of exposure. There is much to be learned about this novel coronavirus. Only time will tell if it disappears from the human population, as SARS did in 2004, or becomes a seasonal disease like flu.
https://theconversation.com/how-does-the-coronavirus-test-work-5-questions-answered-133118
Found at Abnormal Returns Blog www.abnormalreturns.com